Authors: Zeija Cruz | Zian Garcia | Ayesha Pille | Shanne Sy | Micah Ponce | Wynn Romero
AUDIENCE: GRADE 11 / 12 students
TABLE OF CONTENTS
- WHAT IS INTERMOLECULAR FORCES
- TYPES OF INTERMOLECULAR FORCES AND ITS EXAMPLES (5)
- VIDEO LINKS THAT CAN PROVIDE MORE EXAMPLES OF INTERMOLECULAR FORCES
- REFERENCES
WHAT IS INTERMOLECULAR FORCES?
The intermolecular forces are the attractive forces between neighbor molecules. These forces usually exist between molecules, not between atoms. All the intermolecular forces are electrostatic in nature. It is responsible for the solid, liquid, and gas in molecular compounds. It exists only in nonmetals.
We call the forces that hold molecules together intramolecular forces. The interaction of molecules is mediated by a force we call the intermolecular force. forces acting between nearby particles which may be repulsive or attractive. They are not as strong as the intramolecular forces holding a molecule together. The four intermolecular forces are hydrogen bonding, the London dispersion force, ion-ion interactions and dipole-dipole interactions.
Intermolecular forces are electrostatic interactions between permanently or transiently charged chemical species. Intermolecular forces are the attractive or repulsive forces between atoms, molecules, or ions. They are very much weaker than the chemical bonds that hold the atoms together within a molecule, but still they can significantly affect the properties of matter.
For example, intermolecular forces can provide detailed information on the melting and boiling points of a substance, its solubility, and viscosity. Their strength is related to types of molecules and the distance between them. Understanding the different intermolecular forces will help us explain and also predict many physical properties of many substances. It usually designates only the attractive interactions, which hold molecules and ions together in condensed phases-liquids and solids. These forces determine many of the bulk physical properties of matter and mixtures, such as melting point, boiling point and surface tension.
THERE ARE FIVE (5) TYPES OF INTERMOLECULAR FORCES;
- Ion-ion interactions
- dipole-dipole interactions
- Hydrogen bond
- London dispersion force
- Ion-dipole interaction
What is Ion-ion interactions?
Ion-ion interactions are an attractive force between ions with opposite charges. They are also referred to as ionic bonds and are the forces that hold together ionic compounds. Like charges repel each other and opposite charges attract. These Coulombic forces operate over relatively long distances in the gas phase.
Ion-ion forces, also known as ionic bonding, are the simplest to understand. These forces arise from the electrostatic attraction between two ions with opposite charges. They are not technically considered intermolecular forces, but are a helpful starting point for understanding the true IMFs (intermolecular forces).
ex. Solid salt crystals are created when sodium cations in a saturated sodium chloride solution draw in more ions than the solution can hold. Ion-ion interaction is demonstrated by precipitation processes, which create solids by mixing two aqueous solutions.
What is dipole-dipole interactions?
Attractive forces between the positive and negative ends of two polar molecules are known as dipole-dipole forces. Five kJ to twenty kJ per mole are the strengths of dipole-dipole forces. Compared to ionic or covalent bonds, they are far weaker and only really matter when the molecules are in close proximity to one another (touching or virtually touching). Two dipolar molecules interacting over space give rise to dipole-dipole interactions. This happens because the partially positive component of the second polar molecule is drawn to the partially negative region of one of the polar molecules.
Many important physical and biological phenomena, including the high boiling point of water, are explained by this kind of molecular interaction. Diapole-dipole interactions, or dipole-dipole forces, are the electrostatic forces that exist between two permanent polar molecules. The positive end of one molecule typically attracts the negative end of another. As a result, the two molecules get closer, which increases the stability of the material. This contact is different from a conventional ionic or covalent connection since there is no transfer or exchange of electrons.
Dipole-dipole interaction results from an uneven electron distribution within a molecule. The electrons collect at one end of the molecule. The outcome is that the molecule becomes polar, gaining a partially positive charge on one end and a partially negative charge on the other. Nature draws two polar molecules with opposing charges toward one another.
ex. There are dipole-dipole interactions in HCl molecules. Due to the electronegative nature of chlorine when compared to hydrogen, it assumes a partial negative charge while hydrogen assumes a partial positive charge. Then, the HCl molecules undergo a dipole-dipole interaction.
What is Hydrogen bond?
The hydrogen bond has no electrons shared because the hydrogen atom has already sharing its electron with anothor atom. The new atom, that is in the hydrogen bond is remains in electrostatic atom with that hydrogen atom. The other atom of pair, also typically F,N, and O has unshared electron pair which give it a slight negative charge.
The hydrogen bonding, it is interaction involving a hydrogen atoms that is located between pair of other atom with high affinity for electrons, such a bond is weaker than ionic bond and covalent bond but it is stronger than Van der Waals forces.
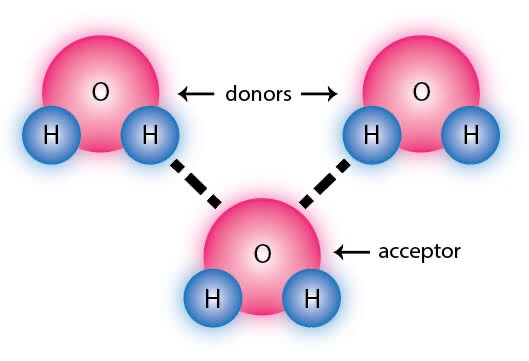
ex. The interaction of the hydrogen, H of a water molecule, with oxygen, O of another water molecule, is an example of hydrogen bonding.
What is London dispersion force?
These are forces that arise due to instantaneous dipoles produced in atoms as well as molecules are known as London Dispersion Forces. LDFs is an Intermolecular force that exists in all molecular whether polar or not. It happens from the changes in the distribution of electrons in a substance that are temporary in nature.
London dispersion forces are a particulare form of the intermolecular forces, which are categorised as the van der Waal forces, that are due to transient changes in electron distribution in the atoms or molecules. Such fluctuations are in a way momentary, throwing into a dipole state the surrounding or at least the close-by particles and thus having the effect of attrition. Alone they perhaps do not appear to be quite powerful, nevertheless, in larger molecules or at lower temperatures, they constitute part of the forces in substances.
ex. This is the London dispersion forces for one helium atom causing a dipole on another nearby helium atom. Helium is a noble gas which by definition only has the weakest of intermolecular forces which are dispersion forces which explains its very low boiling point.
What is Ion-dipole interaction?
One kind of intermolecular force that happens between a charged ion and a polar molecule is called an ion-dipole interaction. Ionic chemicals usually interact in this way when they dissolve in polar liquid/s. The charge of ion or the strength of the dipole in polar molecule enhances the strength of ion-dipole interaction. Positively charged ions known as cations are drawn to the partially negative end of polar molecules. Anions are negatively charged ions that are drawn to a polar molecule’s partially positive end. Dipole molecules naturally interacts with ions, with cations getting pulled to the negative side and anions to the positive side of the polar molecule due to their modest positive and negative charges on different sides.
ex. Ion-dipole forces exist between Na+ and H2O because Na+ forms intermolecular interactions with the oxygen in H2O which is negative due to its different electronegativity than the hydrogen ions, which have a positive end.
VIDEO LINKS;
• https://youtu.be/xCDtuH1OFug?si=j0aEvtI4rubXHau5
• https://youtu.be/LeXCbIDBfQ8?si=2so_BxFFbLF96Qme
https://youtu.be/9YwdeEDrfPI?si=EMk6pDf3jEk9QLxL
https://youtu.be/kOVa3IgzryQ?si=WAF5MwNkZg20CAuX
REFERENCES:
•https://brilliant.org/wiki/ion-ion-interactions/
•https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Specific_Interactions/Ion_-_Ion_Interactions
